Nplate (romiplostim) for immune thrombocytopenia
Last updated July 9, 2025, by Lindsey Shapiro, PhD

What is Nplate for immune thrombocytopenia?
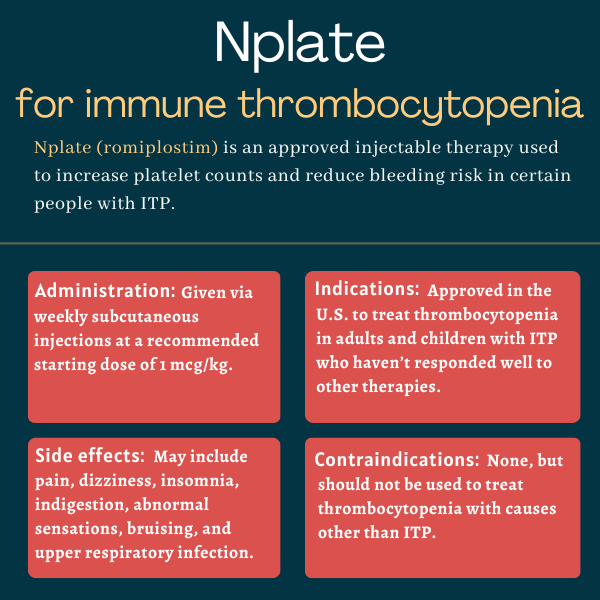
Nplate (romiplostim) is an approved therapy that’s used to treat thrombocytopenia, or low platelet levels, in adults and children with immune thrombocytopenia (ITP) who haven’t responded well to other treatments.
Given via weekly subcutaneous (under-the-skin) injections, it is designed to increase platelet levels and lower the risk of bleeding.
Platelets are the cell fragments needed for blood clotting, and people with ITP have low platelet counts due to erroneous immune attacks, leaving them vulnerable to excessive bleeding.
Nplate works by mimicking the activity of a naturally occurring hormone called thrombopoietin that’s important for stimulating platelet production in the bone marrow. This is expected to increase platelet counts to a level where the risk of clinically significant bleeds is reduced.
The treatment was developed by Amgen and is also approved to increase survival in some people who have been exposed to high amounts of radiation. It is also being investigated for the treatment of chemotherapy-induced thrombocytopenia.
Therapy snapshot
| Brand name: | Nplate |
| Chemical name: | Romiplostim |
| Usage: | Used to increase platelet levels and lower the risk of bleeding in people with ITP |
| Administration: | Subcutaneous injection |
Who with immune thrombocytopenia can take Nplate?
Nplate is approved in the U.S. to treat thrombocytopenia in certain ITP patients who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy (spleen removal), specifically:
- adults
- children, ages 1 and older, who have had ITP for at least six months.
While there are no specific contraindications for Nplate’s use, it should only be used by ITP patients whose platelet levels are low enough that their risk for bleeding is elevated. It is only intended to increase platelet counts to the point where the bleed risk is reduced and should not be used to completely normalize platelets because doses that are too high could lead to potentially life-threatening blood clots.
Nplate also should not be used to treat myelodysplastic syndrome or any other cause of thrombocytopenia other than ITP.
Nplate is also approved to treat ITP in several other regions around the world, although indications may vary. In Canada, for example, it is only approved for adults who fail to respond or cannot tolerate other ITP treatments.
How is Nplate administered in immune thrombocytopenia?
Nplate is given via weekly subcutaneous injections, which are administered by a healthcare provider.
The recommended starting dose for both adults and children with ITP is 1 microgram (mcg) per kilogram (kg) of body weight, which can then be adjusted based on platelet count response to achieve the lowest dose possible that reduces the risk of bleeding.
A healthcare provider will always tell patients if and when their dose of Nplate should be adjusted. If platelet counts do not increase to a level that would avoid clinically important bleeding after a month at the highest possible dose of 10 mcg/kg, Nplate should be discontinued.
Nplate in immune thrombocytopenia clinical trials
Several trials supported the approval of Nplate in adults and children with ITP who failed to respond to prior therapies.
Its use in adults was backed by four studies:
- A pair of placebo-controlled Phase 3 trials tested Nplate in more than 120 patients who had completed at least one prior treatment and had (NCT00102323) or had not (NCT00102336) undergone a splenectomy. Results showed that treatment with Nplate for about six months significantly outperformed a placebo for achieving a durable platelet response. Clinically significant bleeds occurred more often with the placebo than with Nplate (34% vs. 15%).
- An open-label Phase 2 trial (NCT01143038) tested Nplate in 75 adults newly diagnosed with persistent ITP who had an insufficient response to first-line therapies such as corticosteroids and immunoglobulins. Results showed that 93% of patients achieved a platelet response, and about a third achieved disease remission, where a platelet response was maintained for at least six months without any treatment.
- An open-label long-term extension study (NCT00116688) enrolled patients who had participated in other Nplate studies. Most patients achieved a platelet response that was sustained over time.
Nplate’s benefits in pediatric ITP patients 1 year and older were demonstrated in two placebo-controlled trials and a long-term study:
- A placebo-controlled Phase 3 trial (NCT01444417) tested six months of Nplate treatment in 62 children who had failed to respond to previous therapies. Nplate outperformed a placebo for achieving overall and durable platelet responses. Similar benefits were seen in another Phase 1/2 clinical trial (NCT00515203).
- A long-term Phase 3 study (NCT02279173) investigated up to three years of treatment in 203 children diagnosed with ITP at least six months before enrollment. Data showed that Nplate led to sustained platelet responses and reductions in bleeding events, and was well tolerated in the long-term.
Common side effects of Nplate
In adults, the most common side effects of Nplate include:
- pain in the joints, muscles, extremities, abdomen, or shoulders
- dizziness
- insomnia
- indigestion
- abnormal sensations such as tingling, numbness, or prickling (paresthesia).
The most common side effects of Nplate in children include:
- bruising
- upper respiratory tract infection
- sore throat.
Other rare but potentially serious side effects that could occur with Nplate include:
- Blood clots and related complications, including heart attack, stroke, and clots in the liver, legs, or lungs. Dose adjustment guidelines should be carefully followed to reduce the risk of dangerous blood clots.
- Lack of or loss of response to Nplate, including worsening thrombocytopenia, due to neutralizing antibodies developed against the therapy or other causes.
Bleeding Disorders News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Disease flare-ups are a regular part of my life with chronic illness
- Delayed response to Cablivi often tied to other causes, new study finds
- US scientists create tool to predict chronic ITP in children
- US supporters urged to ‘Go RED’ this Bleeding Disorders Awareness Month
- Red blood cell therapy may promote immune tolerance in iTTP: Study
Related articles
-

-

THROMBOTIC THROMBOCYTOPENIC PURPURA
NewsDelayed response to Cablivi often tied to other causes, new study finds
-
-
THROMBOTIC THROMBOCYTOPENIC PURPURA
NewsRed blood cell therapy may promote immune tolerance in iTTP: Study
-