Alvaiz (eltrombopag) for immune thrombocytopenia
Last updated July 14, 2025, by Lindsey Shapiro, PhD

What is Alvaiz for immune thrombocytopenia?
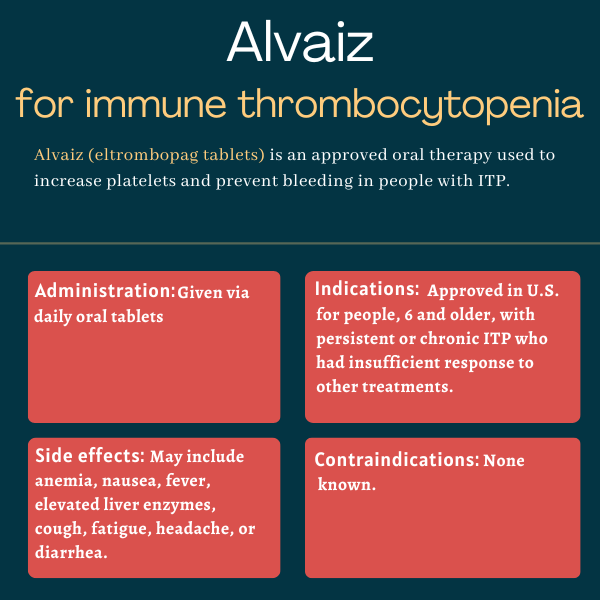
Alvaiz (eltrombopag tablets) is an approved oral therapy used to increase platelet levels and reduce the risk of bleeding in people with immune thrombocytopenia (ITP) who haven’t responded adequately to prior therapies.
In people with ITP, platelets, a blood component critical for blood clotting, are attacked and destroyed by the immune system. Alvaiz is a thrombopoietin receptor agonist that works to increase platelet levels by mimicking the activity of a hormone called thrombopoietin that naturally stimulates platelet production in the bone marrow. This should help to lower the risk of bleeding in people with ITP.
The medication, marketed by Teva Pharmaceuticals, offers a pharmaceutical alternative to the approved ITP therapy Promacta, potentially coming at a lower cost. While both medications contain eltrombopag as the active ingredient, they are not equivalent.
Alvaiz is also approved for treating low platelet counts in certain adults with chronic hepatitis C infections or severe aplastic anemia, another rare blood disorder.
Therapy snapshot
| Brand name: | Alvaiz |
| Chemical name: | Eltrombopag |
| Usage: | Used to increase platelets and reduce bleeding risk in people with ITP |
| Administration: | Oral tablets |
Who with immune thrombocytopenia can take Alvaiz?
Alvaiz is approved in the U.S. for people, 6 and older, with persistent or chronic ITP who have insufficiently responded to one of the following:
- corticosteroids
- immunoglobulins
- spleen removal (splenectomy).
It should only be used for ITP patients whose platelet counts and clinical condition indicate they are at an increased risk for bleeding.
There are no contraindications for using Alvaiz, but it does come with a boxed warning that it could lead to severe or life-threatening liver toxicity in some people. Alvaiz should be avoided in people with myelodysplastic syndrome, a condition that can have some clinical overlap with ITP, because it could increase the risk of blood cancer.
How is Alvaiz administered in immune thrombocytopenia?
Alvaiz is taken as once-daily oral tablets that should be swallowed whole. For most patients, Alvaiz should be started at a dose of 36 mg taken by mouth once daily, and the maximum daily dose should not exceed 54 mg.
People with liver problems or those of East or Southeast Asian descent may need a lower starting dose of 18 mg once daily. A healthcare provider will adjust the dose as needed to achieve a platelet count that is sufficient to reduce the risk of clinically significant bleeds. It is not intended to completely normalize platelet levels.
The tablets should be taken:
- without a meal or with a meal low in calcium
- at least two hours before or four hours after any medications or products containing polyvalent cations, including antacids, calcium-rich foods, and mineral supplements.
Alvaiz is not substitutable with other products that contain eltrombopag on a milligram-to-milligram basis. Patients who may be transitioning from another product in this class should always discuss it with their healthcare provider.
Alvaiz in immune thrombocytopenia clinical trials
The approval of Alvaiz was largely supported by clinical trials of Promacta.
- A Phase 2 trial (NCT00102739) and a Phase 3 trial called RAISE (NCT00370331) both demonstrated oral eltrombopag could increase platelet counts to the point where bleeding risk was reduced in adults with ITP, leading to higher odds of a platelet response than a placebo. The benefits were also sustained long term in an extension study called EXTEND (NCT00351468).
- The Phase 2 PETIT study (NCT00908037) and the Phase 3 PETIT2 trial (NCT01520909) showed similar benefits in children with ITP, where the treatment increased platelet counts and reduced clinically significant bleeding.
Other studies were performed to compare the pharmacological properties of Alvaiz with those of Promacta.
Common side effects of Alvaiz
Across all indications, the most common side effects of Alvaiz include:
- anemia, or lack of healthy red blood cells
- nausea
- fever
- elevated liver enzymes
- cough
- fatigue
- headache
- diarrhea.
In placebo-controlled trials involving children with ITP, the most common side effects were:
- upper respiratory tract infections
- the common cold
- cough
- diarrhea
- fever.
Other less common but potentially serious side effects can happen in people with ITP who are treated with Alvaiz.
- Liver function will be monitored before patients start Alvaiz and during treatment, due to the potential for liver toxicity.
- There’s a risk of blood-clotting complications with Alvaiz, especially if platelet levels get too high. Dose adjustment guidelines must be carefully followed to maintain target platelet counts.
- New or worsening cataracts, or a clouding of the eye’s lens that can affect vision, are possible.
Alvaiz may need to be discontinued if there are signs of liver problems or if platelet levels get too high. It should also be stopped if the platelet count does not reach a sufficient level after a month of using Alvaiz at its maximum daily dose. Bleeding could get worse when Alvaiz is discontinued.
Women who are lactating are advised not to breastfeed during treatment.
Bleeding Disorders News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- US scientists create tool to predict chronic ITP in children
- US supporters urged to ‘Go RED’ this Bleeding Disorders Awareness Month
- Red blood cell therapy may promote immune tolerance in iTTP: Study
- Epstein-Barr virus infection triggers severe ITP in woman, 18: Report
- Veyvondi effective, reduces costs, as VWF replacement therapy: Study
Related articles
-

-
THROMBOTIC THROMBOCYTOPENIC PURPURA
NewsRed blood cell therapy may promote immune tolerance in iTTP: Study
-
-
-

THROMBOTIC THROMBOCYTOPENIC PURPURA
NewsBrazil study shows early diagnosis is key to better outcomes in TTP



