Doptelet (avatrombopag) for immune thrombocytopenia
Last updated July 29, 2025, by Lindsey Shapiro, PhD

What is Doptelet for immune thrombocytopenia?
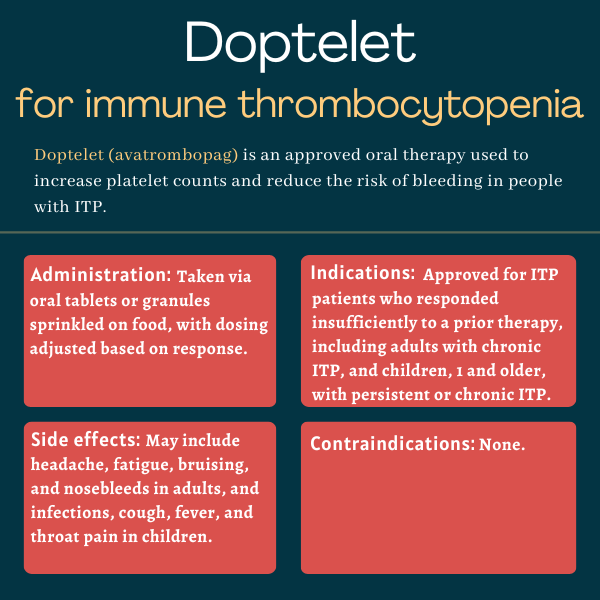
Doptelet (avatrombopag) is an approved oral therapy from Sobi that’s used to increase platelet levels and lower the risk of bleeding in children and adults with immune thrombocytopenia (ITP) who have responded insufficiently to other treatments.
It belongs to a class of ITP therapies called thrombopoietin receptor agonists, which increase platelet levels by mimicking the function of a hormone, called thrombopoietin, that normally stimulates the bone marrow to make more of them. This should help lower the risk of significant bleeding for people with ITP, who don’t have enough platelets, or the cell fragments key for blood clotting, due to autoimmune attacks that destroy them.
Doptelet originally was available only as oral tablets to be swallowed whole. Now, a granulated formulation called Doptelet Sprinkle, which can be sprinkled on food, is also available.
Unlike other medications in its class, Doptelet does not require active liver monitoring, nor come with food restrictions when taking a dose. It is also approved for adults with chronic liver disease who are scheduled to undergo a procedure.
Therapy snapshot
| Brand name: | Doptelet |
| Chemical name: | Avatrombopag |
| Usage: | Used to increase platelets and lower bleeding risk in children and adults with ITP |
| Administration: | Oral tablets or granules |
Who with immune thrombocytopenia can take Doptelet?
In the U.S., Doptelet is approved for certain individuals with ITP who have had an insufficient response to a previous treatment. Eligible patients are:
- adults with chronic ITP
- children, ages 1 year and older, with persistent or chronic ITP
There are no contraindications for its use.
Doptelet is also approved in the European Union for adults with chronic ITP who haven’t responded to other therapies.
How is Doptelet administered in immune thrombocytopenia?
Doptelet is taken orally with food. The specific formulation depends on a person’s age:
- Doptelet oral tablets are available for patients ages 6 and older.
- Doptelet Sprinkle may be used by patients ages 1 to younger than 6 years.
The oral tablets should be swallowed whole.
Doptelet Sprinkle comes in capsules that should be opened up, and the contents mixed in a small amount of soft food or liquid. It can be stirred into applesauce, strawberry jelly, yogurt, milk, orange juice, unflavored pediatric electrolyte solution, or water, among other options. The mixture should be consumed immediately. Capsules should never be swallowed whole, and granules shouldn’t be chewed or crushed.
The starting dose is usually 20 mg once daily for Doptelet tablets and 10 mg daily for Doptelet Sprinkle. The dose may be adjusted by a healthcare provider to maintain the desired platelet count.
The goal is to achieve a platelet level that lowers the risk of bleeding, but Doptelet should not be used to normalize platelets. This should be monitored throughout treatment. Doptelet may need to be discontinued if the platelet count gets too high at the lowest possible dose, or if it remains too low at the maximum dose.
Some other medications may affect the way Doptelet is absorbed in the body, which can influence the necessary dose. Patients should always tell their doctor about any medications they’re using.
Doptelet in immune thrombocytopenia clinical trials
Doptelet’s approvals for ITP in the U.S. were largely supported by data from two Phase 3 clinical trials: one involving previously treated adults with chronic ITP and the other involving previously treated children with primary ITP that had lasted at least six months. Both demonstrated that the therapy was able to sustainably raise platelet counts.
- In Amendment 02 (NCT01438840), adults treated with Doptelet had a significantly longer platelet response — where platelet counts reach a threshold that should lower the risk of bleeding — without the need for rescue therapy, compared with those on a placebo. Nearly two-thirds of people on Doptelet experienced a platelet response within eight days, and the responses were sustained long term.
- In AVA-PED-301 (NCT04516967), Doptelet similarly led to higher rates of platelet responses and durable platelet responses versus a placebo in children, with a good safety profile.
Common side effects of Doptelet
In adults with chronic ITP, the most common side effects of Doptelet are:
- headache
- fatigue
- bruising
- nosebleeds
- upper respiratory tract infection
- joint pain
- bleeding gums
- purple or red spots on the skin (petechiae)
- the common cold.
In children with persistent or chronic ITP, the most common side effects include:
- viral infections
- the common cold
- cough
- fever
- throat pain.
Doptelet also comes with a warning that medications in the thrombopoietin receptor agonist class have been associated with blood clot-related complications in people with ITP. Thus, careful monitoring is required with its use.
These complications may be related to increased platelet levels. Dosing changes for Doptelet should always follow established guidelines to avoid platelet levels getting too high.
Bleeding Disorders News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Disease flare-ups are a regular part of my life with chronic illness
- Delayed response to Cablivi often tied to other causes, new study finds
- US scientists create tool to predict chronic ITP in children
- US supporters urged to ‘Go RED’ this Bleeding Disorders Awareness Month
- Red blood cell therapy may promote immune tolerance in iTTP: Study
Related articles
-

-

THROMBOTIC THROMBOCYTOPENIC PURPURA
NewsDelayed response to Cablivi often tied to other causes, new study finds
-
-
THROMBOTIC THROMBOCYTOPENIC PURPURA
NewsRed blood cell therapy may promote immune tolerance in iTTP: Study
-